Raman spectroscopy as a powerful tool for characterization of graphite used in nuclear plants
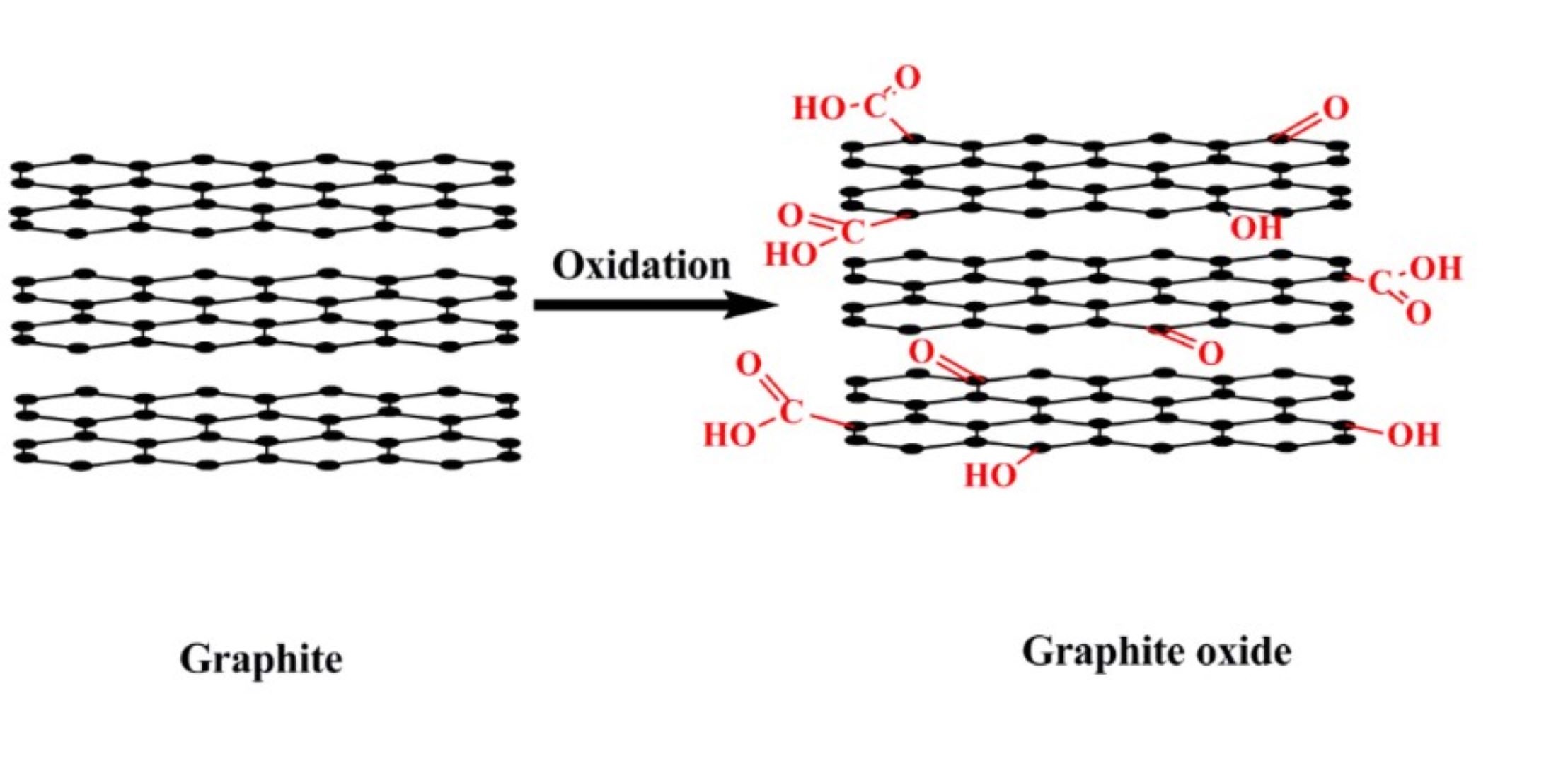
Graphite is one of the most widely used materials in nuclear plants and can also be heavily irradiated over the course of its useful life. In that case the parts made of this material must be disposed of in the nuclear waste repositories. It is normally considered a rather stable material because it can be altered or modified only by resorting to drastic conditions and by very aggressive chemical agents. However, there are two aspects that need to be taken into consideration, as they can be important for environmental contamination, indicating that special attention must also be paid even for nuclear grade graphite. The first is that due to the sintering process required to produce the macroscopic pieces, trace of air could be trapped, because a certain degree of residual porosity is retianed even if it is manufactured to have a relatively high density. This porosity is mainly closed, therefore gases and other substances trapped in can hardly escape. The trapped nitrogen subjected to intense neutron fluxes can lead to the formation of 14C which is an element with a large environmental circulation capacity. Similarly, chlorine may be present which can be activated to 36Cl which similarly can pose a serious radioecological problem because of its great mobility. Both these elements once released can easily enter the chains of ecosystems. The second aspect is in some ways more interesting and concerns the intrinsic reactivity of graphite. When it is stored for very long periods (years or tens of years) it can react with atmospheric oxygen leading to the formation of a rather peculiar compound, the graphite oxide (figure 1). It is a chemical compound of carbon, oxygen, and hydrogen in variable ratios, normally obtained by treating graphite with strong oxidizer. Very aged graphite is no longer a pure material. This is important to be considered because the chemical properties of the oxide are very different, due to a different crystal structure. In the oxide, the distance between the layers increases, while the bonding forces between them decrease. For this reason, very old graphite can exfoliate very easily and release small flakes that can disperse into the environment. This represents an additional risk to be taken into consideration. Furthermore, even the initially closed porosity can become open and the trapped gases can be released to the outside.
Raman spectroscopy is a useful tool for monitoring the chemical-physical characteristics of aged graphite.
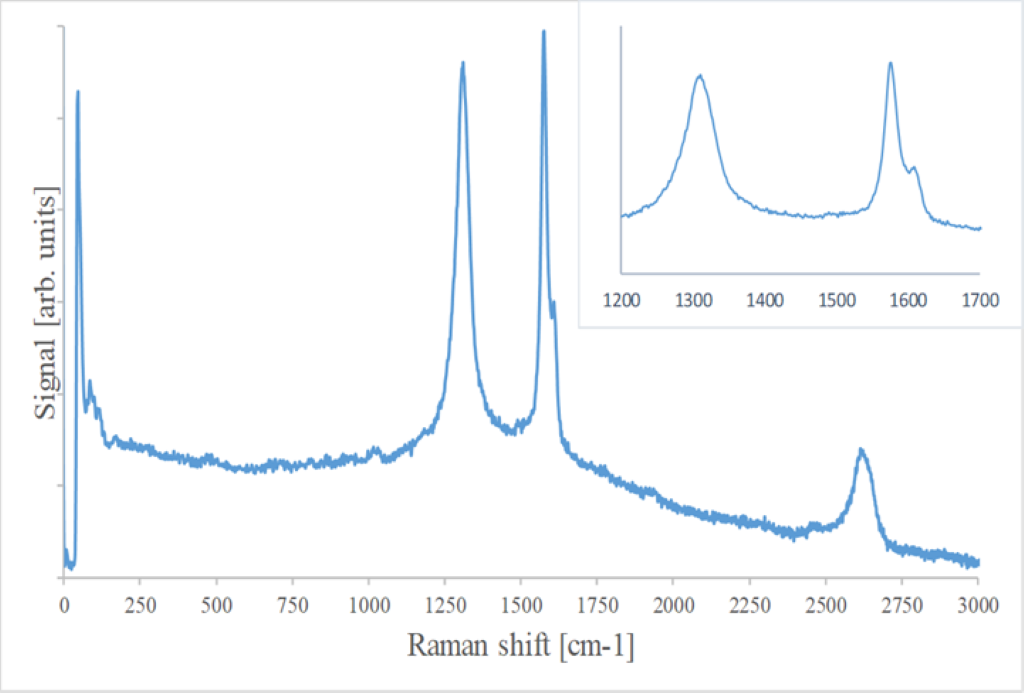
Actually, the spectra of the aged samples (figure 2) are more complicated than the spectra of the pure graphite or of pure graphite oxide, but it turns out to be a combination of the two.
Graphite has a band at about 2650 cm-1 called the D band. Graphite oxide has two characteristic and rather wide bands in the region between about 1200 and 1600 cm-1, which allow a fairly unambiguous identification of this material. On the other hand, graphite has an extremely narrow and sharp Raman band for slightly higher wave numbers, at about 1580 cm-1. In the spectra of the analyzed samples, both signals can be identified even if their relative intensities can vary zone by zone due to the different Raman response of the two phases and due to their different relative quantity. This indicates the presence of both phases in many areas of the samples. It is difficult however to establish the relative ratio between the two phases. Nevertheless, the relative abundance of graphite oxide can be estimated at around 10/15 %. It is more abundant in the external layers and becomes less common toward the center of a macroscopic piece, in good agreement with the oxidation process that starts from the outer layer.
Antonietta Rizzo
ENEA
antonietta.rizzo@enea.it
Alberto Ubaldini
ENEA
alberto.ubaldini@enea.i