Deep geological disposal of radioactive waste in clay rocks
Cement and clays are proposed as sealing materials in underground repositories for radioactive waste. When cement and clay come into contact, chemical gradients between their very different porewater compositions lead to diffusive exchange of solutes, which can result in mineral transformations and alterations of transport properties at the materials interface. Small samples of cement-clay interfaces were prepared and let react over a period of six years.
During this time, the changes in transport properties of the samples were periodically monitored by means of through-diffusion experiments. This technique allows studying the evolution of the diffusive flux across the reacting interface and characterizing the variation of the corresponding effective diffusion coefficient (De) over time.
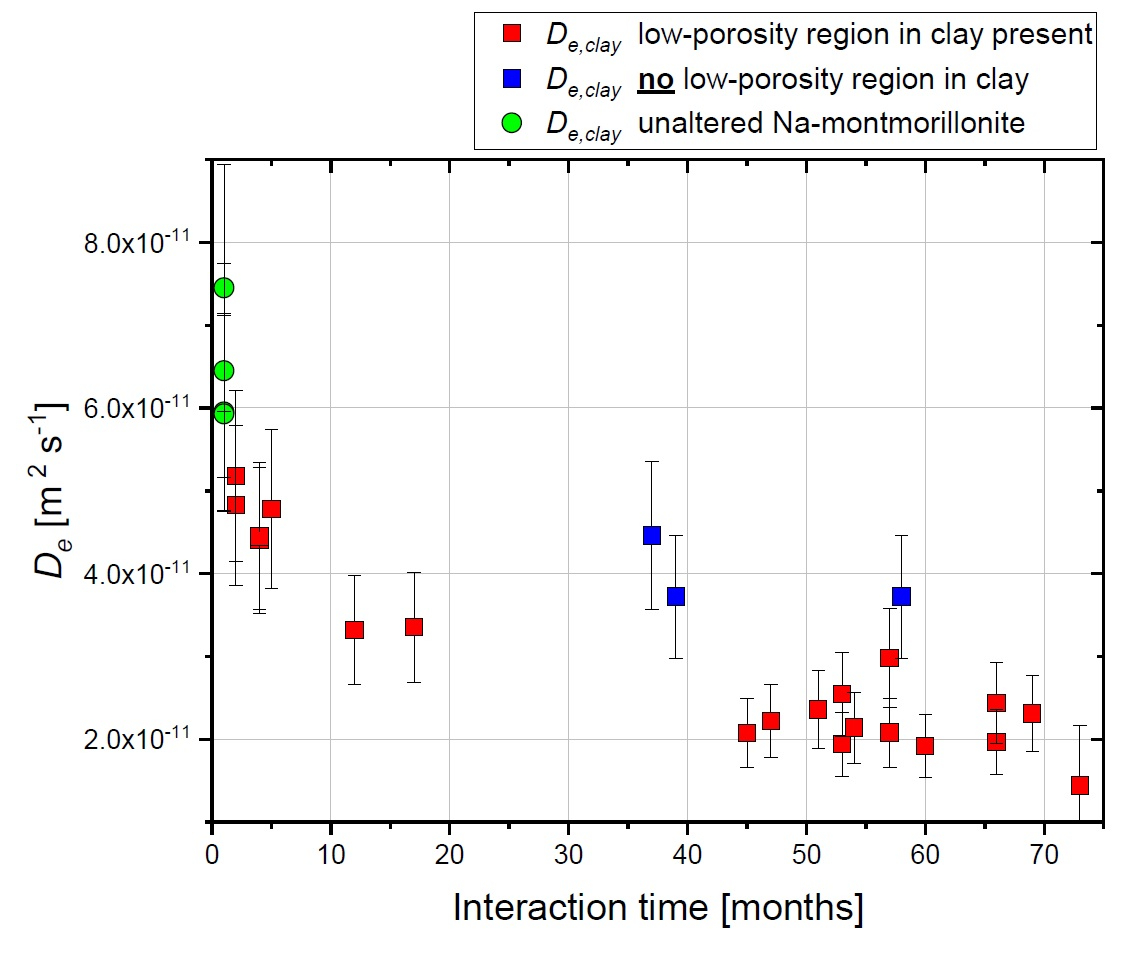
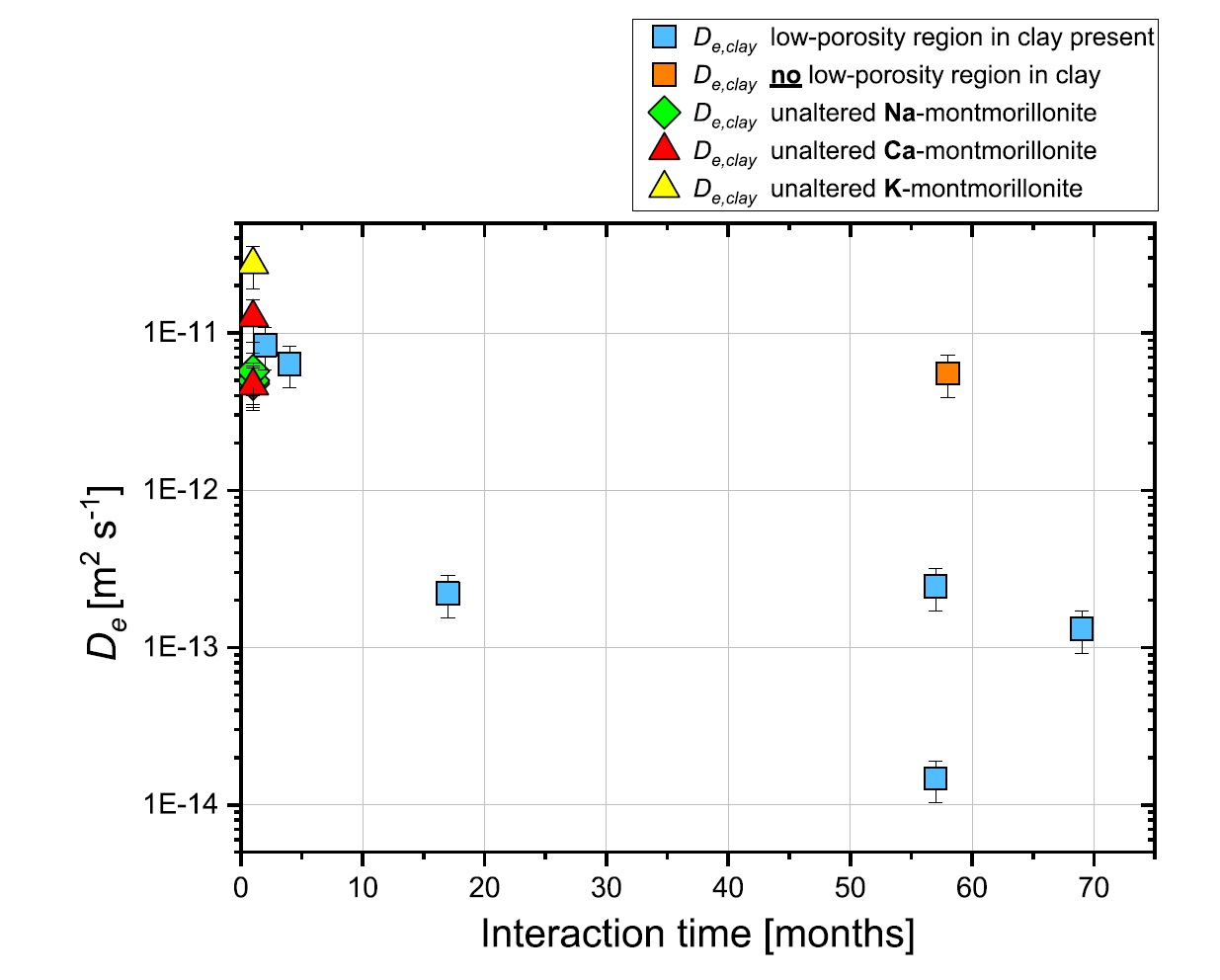
All experiments were performed on samples consisting of a hardened high porosity OPC paste and Na-montmorillonite. These model materials were chosen in order to simplify the mineralogy of the system. The experiments allowed obtaining relevant information regarding the development of the diffusive properties and the reactivity of such a cement-clay interface system. HTO and 36Cl- were used as tracers to study the evolution of both the total and the anion accessible porosity. After six years of reaction a considerable reduction of the flux for both HTO (fig.1) and 36Cl- (fig.2) was observed.
The flux of HTO did, however, not approach zero, which means that connected porosity for diffusive transport of water is still available. The periodic monitoring of the sample evolution showed a strong reduction of the effective diffusion coefficient De of the samples within the first 1.5 years of the experiment and a less prominent decrease in the period between 1.5 and 6 years. The De of 36Cl- showed a stronger reduction compared to that of HTO; for some cells no chloride flux at all could be measured anymore at t > ~4 a.
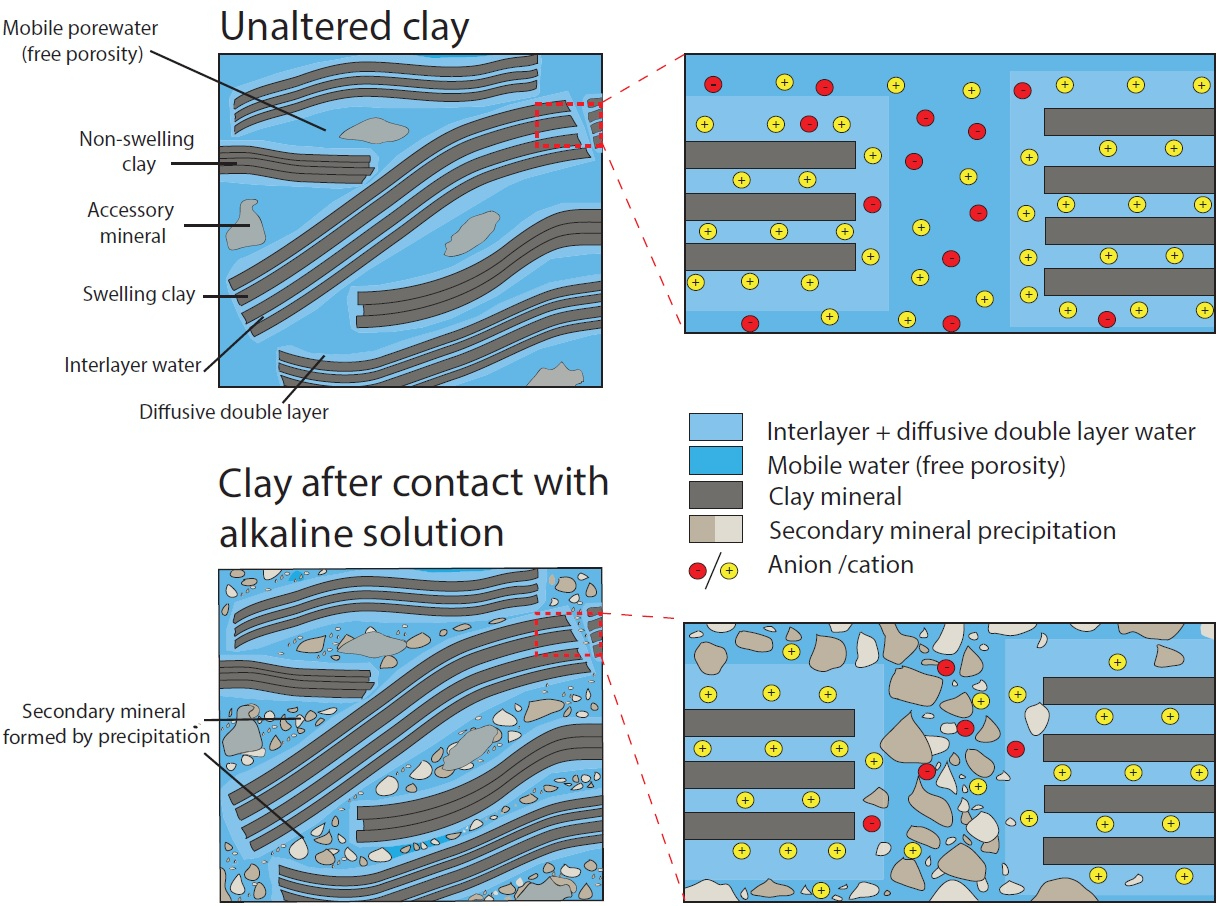
Using additional information on the extension of porosity changes from a neutron imaging study performed in parallel on the same samples (fig. 3), the diffusive properties of each component of the interface, or of a clay zone with reduced porosity could be estimated.
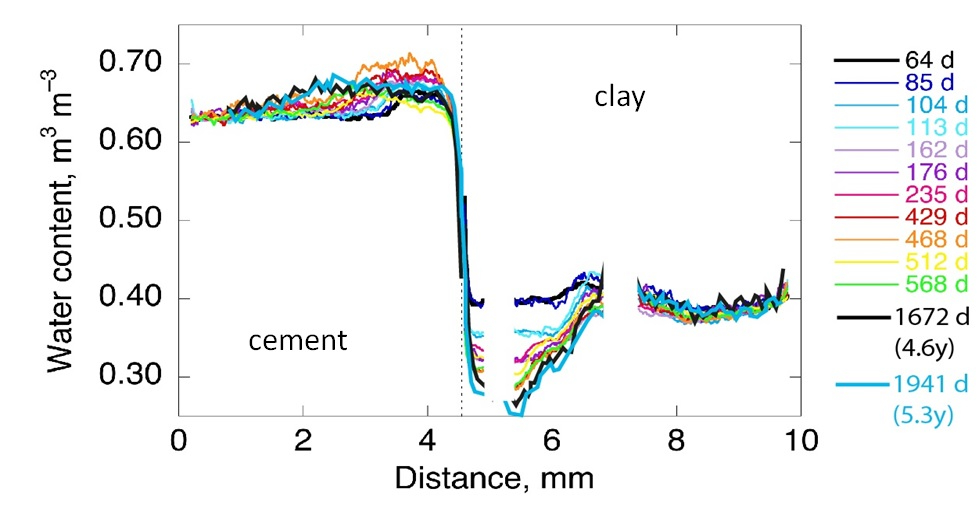 |
Fig. 3: Evolution of profiles of water content with reaction time (in days) across an interface sample. (© PSI) |
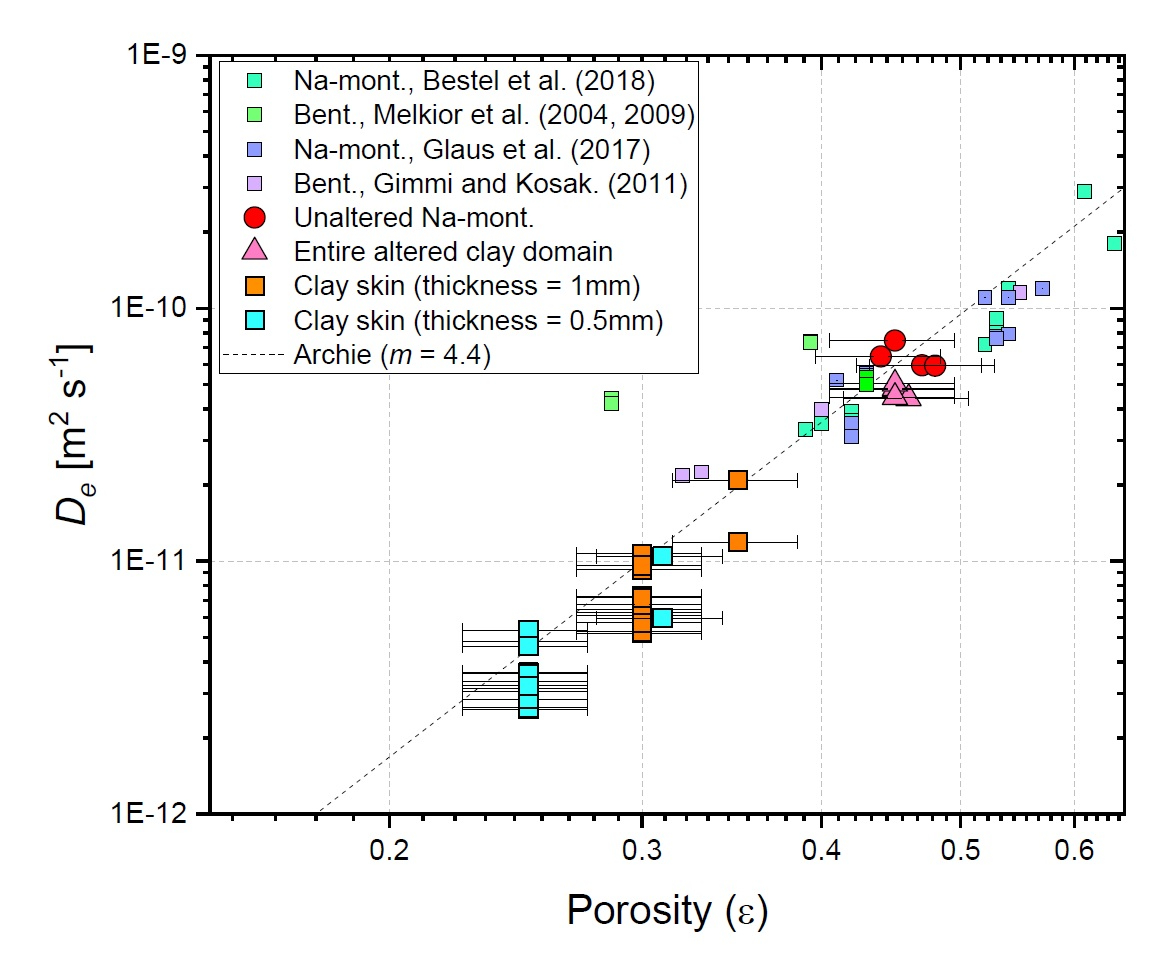
For HTO the relation between the evolution of De and of the porosity in the clay part can be well described with Archie’s empirical law (fig. 4).
For chloride large uncertainties regarding the accessible porosity do not allow a precise correlation.
Whether a complete porosity clogging will take place or some fraction of connected pore space will persist in the sample over reaction times ≫ than 6 years remains an open question (fig 5).
Pietro Luraschi,
Laboratory for Waste Management (LES)
Paul Scherrer Institut
pietro.luraschi@psi.ch